In a new Nature Communications study, researchers from the Medical University of Lodz, Brigham and Women’s Hospital, and Dana-Farber Cancer Institute discovered a method to detect an increased risk of cancer associated with BRCA1 and BRCA2 mutations without using genetic sequencing.
The absence of BRCA1/2 gene mutations is not the basis for the evaluation. Rather, in light of practical changes happen when the pathway those and different qualities direct isn’t working as expected. “For the first time, we have found a signal in the blood to detect pathology associated with increased cancer risk,” says senior author and Dana-Farber researcher Dipanjan Chowdhury, Ph.D.
This new way of detecting risk could become the basis for a method that is more accessible, affordable, and potentially more comprehensive.
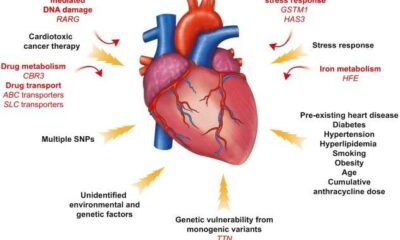
DNA repair is controlled by a broader pathway than the genes BRCA1 and BRCA2. Breast, ovarian, pancreatic, and prostate cancer are more likely to develop as a result of certain mutations in these genes that weaken the repair pathway. In the US, hereditary testing for mutations in these genes may be suggested for individuals with a family background of these tumors. In order to reduce or eliminate their risk of developing cancer, individuals who test positive have the option of undergoing additional screening or surgery.
However, only 10% of the estimated 1 million US carriers of the BRCA1/2 mutation are aware of their status. BRCA mutation testing is not even available in India. A greater number of people may be able to learn that they have an increased risk of cancer through inheritance if more methods of detection become available.
“What if we had an accessible and affordable test for inherited cancer risk that you can get done as an annual physical, the same way we test for diabetes or heart disease risk?” says Chowdhury. “These study results suggest that such a test is in the realm of possibility.”
The presence of a particular group of microRNAs in the blood is the indication of Chowdhury’s increased risk. MicroRNAs are short, non-coding, hairpin-shaped RNA molecules that course in our blood. As disease biomarkers, they are becoming increasingly popular.
A sign that the DNA repair process is actively breaking down is the presence of a specific constellation of microRNAs, similar to the distinct knocking that an engine might make when one of its parts starts to fail. The shortfall of that star grouping is an indication of a functioning DNA repair process — calm like an engine that purrs.
The team looked at blood samples from 653 people from six biorepositories—four in the United States, one in Poland, and one in India—in the proof-of-concept study. About half of the samples were affirmed to have BRCA mutations and half not.
The microRNAs in each sample were identified by the team through next-generation sequencing, and a data set containing the microRNAs and BRCA mutation status for each sample was created. Chowdhury’s colleagues at the Clinical College of Lodz utilized AI to distinguish a mark — that one of a kind heavenly body of microRNAs — seen only in those samples positive for BRCA mutations.
The fact that the model they developed was able to correctly identify the presence of BRCA mutations in 94% of cases is strong evidence that the signal is a novel method for identifying individuals at increased risk of cancer.
The validity of the signal is thoroughly evaluated in this proof-of-concept study, but it does not serve as the foundation for a clinical test that could be used in an annual exam. However, a clinical test at a reasonable cost is attainable.
The microRNA signature can be detected using PCR tests, which use the same technology as COVID-19 infections and can be used in place of next-generation sequencing, making it affordable and accessible. Moreover, says Chowdhury, the precision of the model used to identify the microRNA group of stars will work on as it is prepared using increasingly large datasets.
Chowdhury hypothesizes that the test could also be a more comprehensive indicator of increased risk than genetic sequencing of the BRCA1 and BRCA2 genes because it shows signs of pathology in action, regardless of which gene mutations cause it. At present, hereditary testing catches just known defects in a couple of known qualities in the genome that could interfere with the DNA fix process.
“We are constantly discovering new genes and mutations that could impact this pathway, so it is hard for genetic testing to keep up,” says Chowdhury. “A test that is independent of that, that shows you have a problem in the pathway, could more broadly identify people at risk.”
Chowdhury is currently working on a clinical study based on this work to see if a similar test could be used to screen for early ovarian cancer. The examination is inspired by a past retrospective study recommending that a particular star grouping of microRNA seems when cancer starts.

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago