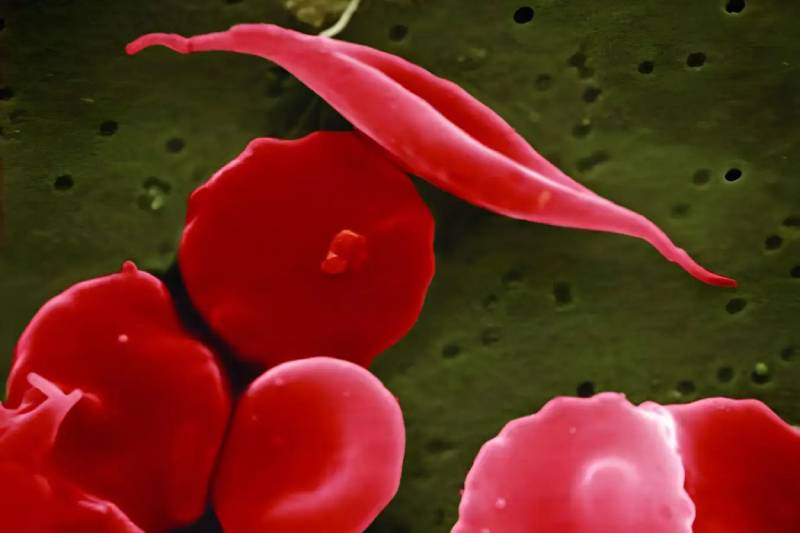
A new treatment for sickle cell disease based on CRISPR gene editing has the potential to completely change the medical, agricultural, and other industries.
The year 2023 marked the breakthrough of CRISPR gene-editing, as it emerged from the laboratory and gained traction in the public and American medical systems. Casgevy, sometimes known as exa-cel, is a sickle cell disease treatment developed by CRISPR Therapeutics and partner Vertex that was recently licensed by the Food and Drug Administration. It is the first gene-editing CRISPR therapeutic. This follows a similar approval by UK authorities in a momentous occasion for a gene-editing technology whose roots were established in the 1980s and which ultimately led to the 2020 Chemistry Nobel Prize for pioneering CRISPR scientists Jennifer Doudna and Emmanuelle Charpentier.
It is significant that there has been a decades-long lapse between the discovery of a viable treatment for blood diseases like sickle cell disease, which affect hundreds of thousands of people worldwide, and its commercial introduction. If history is any guide, then even more advanced CRISPR gene-editing techniques under research could be used to treat conditions like heart disease, cancer, and muscular dystrophy. They could also be used to produce stronger plants and cattle that can withstand new virus strains and climate change, as well as completely transform the energy sector by modifying bacterial DNA to produce biofuels that will burn cleaner in the next decades. Additionally, creative applications of CRISPR, with help from other technologies like artificial intelligence, may enable even more focused, accurate gene editing, which would hasten further research having ramifications for nearly every sector of the economy that uses biological material, including energy, agriculture, and medicine.
Feng Zhang, another pioneer of CRISPR, a molecular biologist and core member of the Broad Institute of MIT and Harvard, stated that with new CRISPR discoveries driven by AI, “we can expand the toolbox available for gene editing, which is crucial for therapeutic, diagnostic, and research applications… but also a great way to better understand the vast diversity of microbial defense mechanisms.”
These defense mechanisms serve as the foundation for modified CRISPR gene-editing systems, such as the CRISPR-Cas9 system that Casgevy uses to remove the faulty genes that cause sickle cell disease. Bacteria use these mechanisms to fend off enemy pathogens like viruses by snipping away their DNA and mounting an immune response. However, that is just one instance of CRISPR. Numerous more methods, ranging from those already being explored in clinical settings to those yet to be identified by bacteria in diverse settings, may open up new possibilities for scientists seeking to modify genes for industrial, medical, or agricultural purposes.
“The Cas9 system, which is the one that is most advanced in terms of clinical applications, is just one of many different types of CRISPR systems. Each one has unique attributes that may be useful in different applications,” Zhang said.
Here’s where algorithmic boosts from AI can help scholars. Zhang and a group of researchers from the National Center for Biotechnology Information at the National Institutes of Health, the McGovern Institute for Brain Research at the Massachusetts Institute of Technology, and the Broad Institute announced in November that they had found a “treasure trove” of novel CRISPR systems. The researchers searched genomic databases of bacteria found in everything from mines to breweries to dog saliva using a method they called FLSHclust, or Fast Locality-Sensitive Hashing-based clustering. They found 188 different kinds of uncommon, previously unidentified CRISPR systems in bacteria, which may lead to more accurate, focused, and safe gene-editing techniques in human medicine and other fields.
“One of the important lessons we took from this study is the importance of biodiversity,” Zhang said. “The new systems that we found came from environmental samples from around the world. Within these samples, we discovered lots of new, exciting biology, and, hopefully, the seeds for future powerful tools and therapeutics.”

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 days ago
Diabetology2 days ago