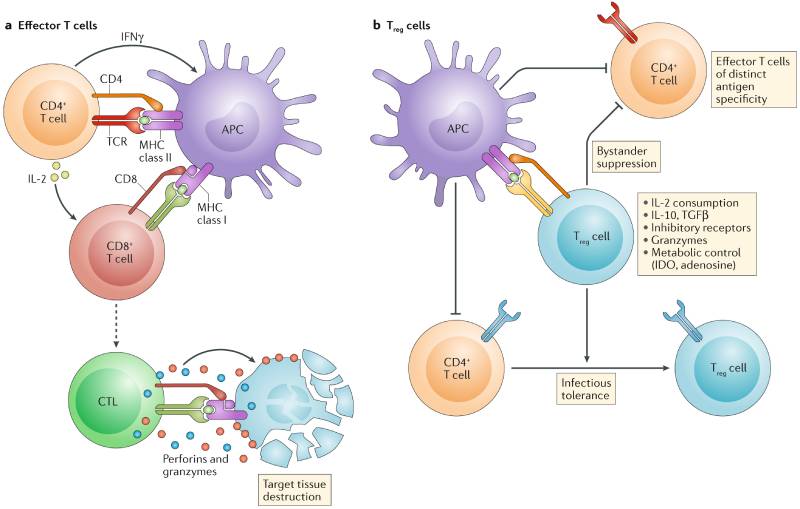
Safe designated spot bar (ICB) has restricted progress in non-little cell cellular breakdown in the lungs (NSCLC) patients. T cells are stimulated by ICB to kill tumors; However, immunosuppressive mechanisms frequently dampen anti-tumor responses. Dykema et al. investigated how anti-PD-1 treatment-induced anti-tumor immunity is bolstered by regulatory T cells (Treg). By coordinating single cell TCRseq/RNAseq from human NSCLC patients and murine growths, various cancer Treg subclusters were distinguished. An “enacted” subcluster communicating TNFR superfamily qualities OX40 and GITR was exceptionally suppressive and related with ICB opposition. In murine growths most of cancer receptive Treg separated into a proinflammatory Th1-like aggregate, and this Th1-like subcluster was improved in human enemy of PD-1-responsive lung growths. These discoveries distinguish variety inside cancer Treg and recommend that focusing on unambiguous subclusters could further develop reactions to ICB. — Hannah Isles
Administrative White blood cells (Treg) are traditionally seen as silencers of endogenous and treatment initiated antitumor resistance; be that as it may, their part in regulating reactions to safe designated spot barricade (ICB) is muddled. We combined single-cell analysis of tumor-associated antigen (TAA)–specific Treg derived from a murine tumor model with single-cell RNA-seq/T cell receptor sequencing (TCRseq) of more than 73,000 tumor-infiltrating Treg (TIL-Treg) from anti-PD-1–treated and treatment-naive non-small cell lung cancers (NSCLC). We distinguished 10 subsets of human Until Treg, the greater part of which have high concordance with murine Until Treg subsets. Just a single subset specifically communicates elevated degrees of TNFRSF4 (OX40) and TNFRSF18 (GITR), whose engangement by related ligand intervened proliferative projects and NF-κB enactment, as well as numerous qualities engaged with Treg concealment, including LAG3. Practically, the OX40hiGITRhi subset is the most profoundly suppressive ex vivo, and its higher portrayal among complete Until Treg connected with protection from PD-1 barricade. Suddenly, in the murine cancer model, we found that basically all Until Treg-communicating Immune system microorganism receptors that are explicit for TAA completely create a particular TH1-like mark north of a 2-week time frame after section into the growth, down-managing FoxP3 and up-controlling articulation of TBX21 (Tbet), IFNG, and certain proinflammatory granzymes. Move gaining of a quality score from the murine TAA-explicit TH1-like Treg subset to the human single-cell dataset uncovered an exceptionally closely resembling subcluster that was enhanced in enemy of PD-1-answering cancers. These results suggest that TAA-specific TIL-Treg may positively contribute to antitumor responses and that TIL-Treg partition into multiple distinct transcriptionally defined subsets with potentially opposing effects on ICB-induced antitumor immunity.

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago