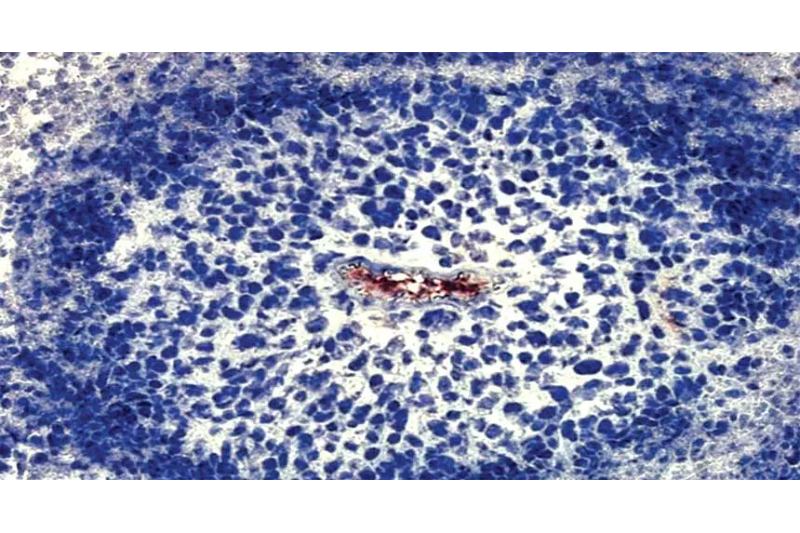
The survival of cancer cells during the initial hours following their detachment from oxygen has been clarified by researchers at the Francis Crick Institute.
This discovery, which was published in The EMBO Journal, may eventually aid in preventing cancer from developing treatment resistance.
Cells use oxygen primarily for the synthesis of energy. Most cells are able to tolerate low oxygen levels because they have the ability to adapt by altering the proteins they generate and using other mechanisms to produce energy than they would at normal oxygen levels. A protein known as HIF1α, which activates gene activity, coordinates this.
Cells exposed to a low oxygen period lack an evident mechanism for continuing energy production, even if HIF1α levels increase as soon as the oxygen supply declines. The necessary genes require roughly 24 hours to create proteins.
Through examining how cancer cells utilize their resources, the scientists discovered that, three hours after the cells are starved of oxygen, a process known as glycolysis—which involves breaking down glucose to produce energy—increases.
When cells are exposed to low oxygen levels on a regular basis, HIF1α has been shown to stimulate enhanced glycolysis. Nevertheless, glycolysis increased even after the researchers genetically altered the cells to stop producing HIF1α and oxygen-deprived them, indicating that additional variables may have contributed to this increase right after oxygen deprivation.
The amount of NAD+, a tiny chemical present in cells and essential to glycolysis, determines how quickly the process proceeds. The group found that in order for glycolysis to increase, LDHA and GOT1 must cooperate to produce adequate NAD+.
This work shows that although LDHA and GOT1 are present in normal oxygen environments, they serve as buffers in low oxygen states. Accordingly, a cell that is exposed to normal oxygen is primed and ready to respond to an abrupt drop in oxygen levels in its surroundings. It doesn’t need to produce new material.
Notably, the researchers discovered that GOT1 activity contributes to the accumulation of HIF1α, via a process for which Crick Clinical Director Peter Ratcliffe won the 2019 Nobel Prize in Physiology or Medicine. Therefore, by guaranteeing strong HIF1α activity, GOT1 can influence the long-term adaptation of cells to oxygen constraint in addition to short-term support for glycolysis.
Potential treatments for cancer
According to the research, blocking LDHA and GOT1 may be able to target treatment-resistant cancer cells that are difficult to reach since they are probably located deep within a tumor where they do not have access to oxygen or a blood supply. This would prevent these cancer cells from producing energy.
By preventing LDHA and GOT1 from acting, the researchers investigated this theory. They discovered that inhibiting both enzymes at the same time killed cancer cells in low oxygen environments more efficiently than targeting either enzyme alone or in normal oxygen levels.
This suggests that LDHA and GOT1 are good targets for treatment, particularly since normal oxygen-supplying cells, such as non-cancerous cells, shouldn’t be impacted because they don’t require these enzymes as much.
How to target cancer cells directly without harming healthy cells is a major challenge in cancer therapy, according to Dimitrios Anastasiou, Group Leader of the Cancer Metabolism Laboratory at the Crick. Instead of focusing on the acute requirements of cells as a result of a changing environment, as is typically the case, researchers have examined how cells adapt to chronic stress. Our findings point to a susceptibility that cancer cells have during the initial few hours of oxygen starvation.”
First author Fiona Grimm, a former Crick doctoral student, stated, “We may conceive of this as a typical supply and demand problem: there is higher demand for LDHA and GOT1 under low oxygen settings than in normal oxygen conditions. We can potentially target oxygen-deprived cells before they adapt to low oxygen and become difficult to reach or resistant to therapy by inhibiting these enzymes where they are most needed.”
In an effort to produce cancer medications that may be used either on their own or in conjunction with other therapies, the lab is currently collaborating with GSK to find tiny compounds that can block the processes that cells utilize to survive in environments where oxygen is scarce.
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology7 days ago
Diabetology7 days ago
 Diabetology7 days ago
Diabetology7 days ago
 Diabetology4 days ago
Diabetology4 days ago
 Diabetology15 hours ago
Diabetology15 hours ago