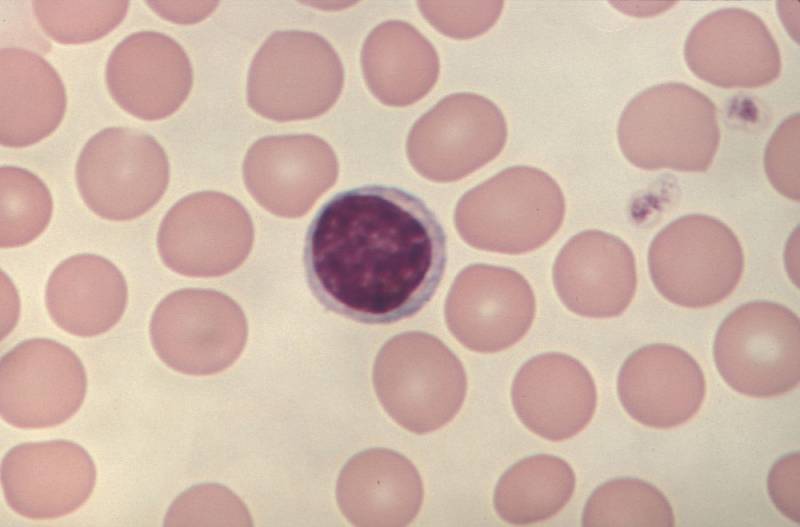
Northwestern Medication specialists have found how the protein PD-1 controls fundamental metabolic cycles in cancer cells to advance disease development in Lymphocyte non-Hodgkin lymphomas (T-NHLs), as per a review distributed in Nature Malignant growth.
“We found that PD-1 controls the metabolism of these T-cell lymphoma cells, and these metabolic changes completely reprogram the epigenetics and transcriptional signatures of the T-cell lymphomas themselves,” said Jaehyuk Choi, MD, PhD, the Jack W. Graffin Professor and associate professor of Dermatology and of Biochemistry and Molecular Genetics.
Jay Daniels, an understudy in the Driskill Graduate Program in Life Sciences (DGP), was co-lead creator of the review.
The PD-1 protein, which is encoded by the PDCD1 quality, is referred to go about as a cancer silencer in T-NHLs, which causes white platelets called lymphocytes to develop strangely and structure growths all through the body.
The malignant growth is exceptionally forceful, and not many treatment choices are accessible because of a restricted comprehension of the instruments that drive PD-1 cancer concealment. Notwithstanding, a new report from the Choi research center observed that expanded PD-1 transformations are really connected with infection movement and unfortunate forecast.
To distinguish these instruments, Choi’s group performed RNA-sequencing and metabolic imaging to concentrate on cancer cells from mouse models of T-NHLs and tests of patient T-NHL growths. Utilizing these strategies, the specialists found that PD-1 limits and directs various fundamental metabolic cycles inside Lymphocyte lymphoma cells, subsequently expanding the forcefulness of the disease cells.
“We had discovered previously there were 86 driver genes and we were surprised to find that this one mutation [in PD-1]seems to completely govern the lymphoma behavior,” Choi said.
The examiners additionally managed a chemical inhibitor compound in the PD1-lacking mice, which forestalled intracellular motioning in the T-NHL cells.
“Our data suggests that there could be a therapeutic opportunity to target specific pathways using FDA-approved drugs specifically only for the PD-1 mutant lymphomas,” Choi said.
Later on, these remarkable sub-atomic highlights could be utilized as rules for deciding the seriousness and phase of T-NHLs and could further develop treatment methodologies for patients, as indicated by Choi.
“We’re also really interested in trying to figure out whether this should be important criteria for determining how to use current therapies and also how to design clinical trials,” Choi added.
Joan Guitart, MD, head of Dermatopathology in the Division of Dermatology, and Calvin Regulation, a DGP understudy, were co-creators of the review.
Choi and Guitart are individuals from the Robert H. Lurie Thorough Malignant growth Focus of Northwestern College.
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology4 days ago
Diabetology4 days ago
 Diabetology7 hours ago
Diabetology7 hours ago
 Diabetology7 hours ago
Diabetology7 hours ago