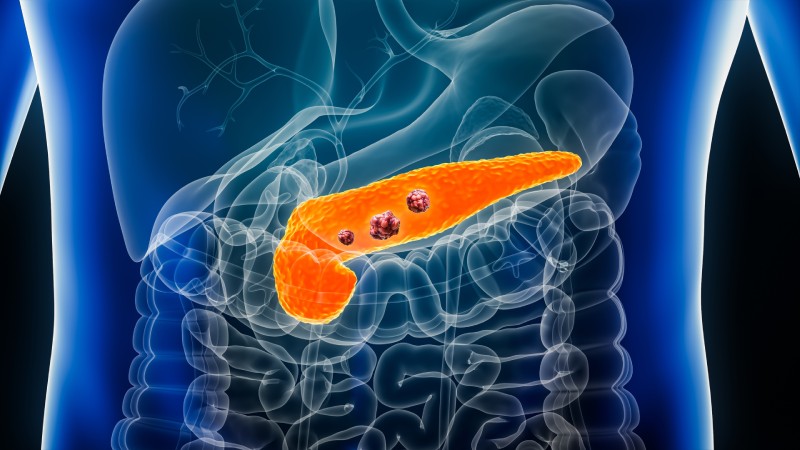
According to new research led by Johns Hopkins investigators, giving patients with operable pancreatic cancers a three-pronged combination immunotherapy treatment consisting of the pancreatic cancer vaccine GVAX, the immune checkpoint therapy nivolumab, and urelemab, an anti-CD137 agonist antibody treatment, is safe, it increases the amount of cancer-killing immune system T cells in the tumours, and it appears effective when given two weeks prior to cancer removal surgery. The journal Nature Communications released an online summary of the work on June 20.
The Bloomberg Kimmel Institute for Cancer Immunotherapy, the Johns Hopkins Kimmel Cancer Centre, and the Johns Hopkins University School of Medicine are leading this study, which is the most recent from a platform trial that was established in 2015 to examine immunotherapy treatments for pancreatic cancer patients both before surgery (neoadjuvant) and after surgery (adjuvant). This format enables researchers to use trial data to advance pancreatic cancer immunotherapy research inside the same study.
Ten patients in this most recent round of the trial received the combo therapy. The median overall survival, or time to death, was 35.5 months, whereas the median disease-free survival, or the amount of time following therapy during which no cancer is diagnosed, was 33.51 months. These were greater than those observed in earlier trial arms that evaluated the pancreatic cancer vaccine both alone and in combination with nivolumab, but due to the limited sample size, the findings lacked statistical significance.
Additionally, the tumour samples examined in the most recent arm had significantly more cancer-fighting immune cells than tumour samples from patients who received either just the vaccine or the vaccine plus nivolumab. According to senior study author Lei Zheng, M.D., Ph.D., co-director of the Pancreatic Cancer Precision Medicine Centre of Excellence and professor of oncology at Johns Hopkins University School of Medicine, results indicate that this therapy combination merits additional research in bigger clinical trials.
According to Zheng, the platform experiment serves two objectives in relation to pancreatic cancer treatments administered during a two-week “window of opportunity” before surgery. The patient’s immune cells can now be taught how to react to tumours by immunotherapies, enabling them to maintain surveillance in the event that the disease returns. Second, it allows researchers to assess the tumours that were surgically removed to see how well the tumours responded to the treatment. The trial’s fourth component is currently investigating anti-interleukin-8 neutrophil-blocking antibodies in pancreatic tumours.

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago