The first over-the-counter continuous glucose monitor (CGM) has received marketing approval from the Food and Drug Administration.
The Dexcom Stelo Glucose Biosensor System is a product that is approved to help both people without diabetes who want to monitor their blood glucose levels and persons with type 2 diabetes who do not use insulin. It will be released in the summer of 2024.
The wearable sensor Stelo has a 15-day lifespan before needing to be changed. Every fifteen minutes, it connects to an app on your phone to provide blood glucose readings. Data demonstrating that the product was just as effective as comparable ones was submitted to the FDA, leading to its approval. Nevertheless, the public cannot currently access the data.
Jake Leach, chief operating officer of Dexcom, expressed his excitement about Stelo’s ability to reach a wider audience. Due to its over-the-counter availability, consumers can avoid some of the cost barriers connected to insurance companies.
“As we look back at milestones, this is the one that is going to be up there on the chart of big changes for the CGM industry. And we couldn’t be more excited that Dexcom is the one leading the charge,” Leach told Verywell.
The product is a great development, according to Pouya Shafipour, MD, a family and obesity medicine physician at Providence Saint John’s Health Center. However, he raised concerns about its accuracy and potential patient reactions to data that is almost real-time.
“There’s a lot of different things that can influence the results such as diet, exercise, supplements, vitamins you’re taking, so for someone who’s not in health care, or doesn’t have expertise, my fear is it might cause a lot of anxiety if they see, suddenly, the blood sugar goes up,” Shafipour told Verywell.
In Whom Is Stelo Suitable?
Patients who are successful with CGMs, according to Shafipour, concentrate on larger patterns rather than isolated spikes or dips. He continued, “A patient with early-onset type 2 diabetes or prediabetes would be the ideal candidate for this product.”
“They can kind of use this as the guidance for future diet and behavior, or exercise management,” Shafipour said.
Along with the rise in interest in sports performance and longevity, CGMs have also become more and more popular.
Although the company is interested in the sporting sector, diabetic patients are Dexcom’s top priority, according to COO Leach.
“I can’t tell you how many people I know that have tried a CGM that don’t have diabetes that say, ‘I’ve learned some interesting stuff. That’s kind of cool, but now what am I supposed to do with this information? How do I actually improve my performance? How do I actually lose weight?’” Leach said. “The products have to be designed for that. Over time, we intend to bring products to serve those use cases. But Stelo is specifically designed for type 2 diabetes.”
What Are the Dangers of Using Stelo?
The FDA states that using Stelo may cause pain, skin irritation, and localized infections.
According to the EPA, because Stelo does not include a hypoglycemia warning system, it is not recommended as a substitute for insulin users. Additionally, users are advised against using the available data to make any significant health decisions without first speaking with a physician.
Shafipour stated he believes there is a market for this gadget even with the possible risks of incorrect use and patients overanalyzing short-term data.
“I think they’re great devices,” Shafipour said. “They can provide a lot of information in terms of activities, different types of foods that can influence [blood sugar levels], and there’s a lot of doctors who have great podcasts that are advocating for these devices for people to take control of their health and diet.”
 Diabetology1 week ago
Diabetology1 week ago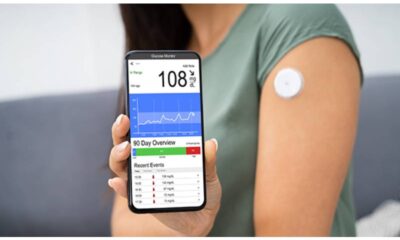
 Diabetology4 days ago
Diabetology4 days ago
 Diabetology11 hours ago
Diabetology11 hours ago
 Diabetology12 hours ago
Diabetology12 hours ago











