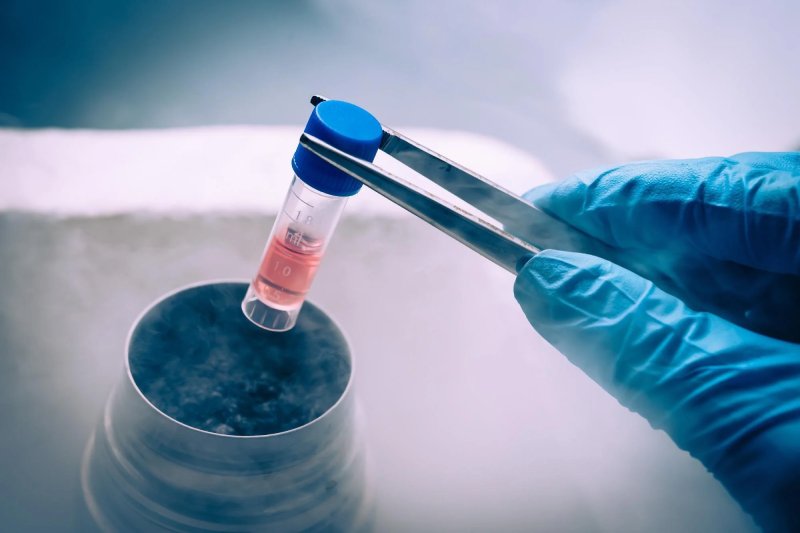
Researchers are now working to maximize the function and potential of stem cell-based treatments for future use in patients, following encouraging results from preclinical research and a recent clinical trial on stem cell-based treatments for Type 1 diabetes.
Recent estimates indicate that Type 1 diabetes (T1D) affects more than 8 billion people worldwide. Due to the immune system’s destruction of beta cells in T1D, the pancreas’ so-called beta cells do not produce enough insulin, which causes blood sugar levels to rise above normal. T1D can result in severe organ damage over time. The standard treatment for T1D is daily insulin injections, but some patients have trouble keeping their blood sugar levels within the normal range and run the risk of developing complications. There is no cure for T1D.
The transplantation of beta cells is one alternative treatment option being looked into for T1D patients. Reports of a recently launched clinical trial (Vertex NCT04786262) have given people hope that stem cell-derived beta cells could become an alternative and renewable treatment for T1D patients, addressing the ongoing scarcity of cadaveric beta cells.
Researchers have made significant progress in the lab in recent years in the generation of large numbers of stem cell-derived beta cells (sBCs). However, the current methods for producing mature, functioning sBCs and removing any remaining residual stem cells that may cause tumors in patients are expensive and labor-intensive, making them difficult to implement on a large scale. A straightforward solution to those problems has now been found in a recent study that was published in the journal Stem Cell Reports. In their work, Holger Russ and partners from the College of Florida, USA, tracked down that isolating and possibly tumorigenic non-BCs can be disposed of from sBC societies by a concise treatment with a chemotherapeutic medication. After transplanting sBCs into diabetic mice, this method not only reduced graft overgrowth and tumor formation but also enhanced their maturation and functionality. As a consequence of this, it is possible that this approach will cut down on the amount of time and money required to produce sBC transplants that are both secure and effective. Additionally, it will likely make a contribution to the improvement of access to this kind of treatment for a greater number of T1D patients in the long run.

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology1 week ago
Diabetology1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago